CHMP positive opinion is based on evidence from the EFFISAYIL 1 trial, the largest clinical trial in patients with generalized pustular psoriasis (GPP) flares1
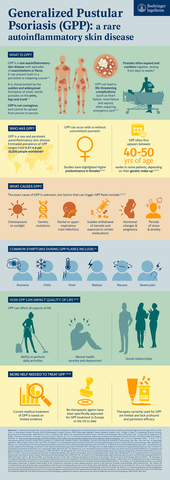
GPP is a rare and potentially life-threatening skin condition distinct from plaque psoriasis2, 3, 4
Monoclonal antibody spesolimab specifically inhibits interleukin-36 (IL-36) signaling5, 6, 7
INGELHEIM, Germany -- (BUSINESS WIRE) --
The European Medicine Agency’s Committee for Medicinal Products for Human Use (CHMP) recommended the granting of a conditional market authorization* for Boehringer Ingelheim’s spesolimab as first in class treatment option for generalized pustular psoriasis (GPP) flares in adults. Spesolimab, marketed in the U.S. and in Japan as SPEVIGO®, is a novel, selective antibody that blocks the activation of the interleukin-36 receptor (IL-36R), a signaling pathway within the immune system shown to be involved in the pathogenesis of GPP.1, 3, 5
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20221014005235/en/
“GPP flares are unpredictable, often require emergency care and can lead to serious, life-threatening complications such as shock and multisystem organ failure,” said Hervé Bachelez, M.D., Ph.D., study investigator and professor at the Department of Dermatology of the Saint-Louis University Hospital in Paris. “The positive opinion for spesolimab brings us one step closer to a new and first treatment option specifically designed to target the IL-36 pathway that is central to the pathogenesis of GPP.”
“This positive recommendation recognizes spesolimab’s potential as a new targeted monoclonal antibody that could treat the underlying cause of GPP. The accelerated development of spesolimab underscores our continued commitment to develop faster and more novel treatments for people with high unmet medical needs,” commented Carinne Brouillon, Member of the Board of Managing Directors, responsible for Human Pharma, Boehringer Ingelheim.
The CHMP’s positive opinion on spesolimab is based on results from the pivotal EFFISAYIL® 1 Phase II clinical trial.1 In the 12-week trial, patients experiencing a GPP flare were treated with spesolimab or placebo. Most patients at the outset of the trial had a high, or very high, density of pustules, and impaired quality of life. After one week, 54% of patients treated with spesolimab showed no visible pustules compared to placebo (6%).1 Adverse events were reported in 66% of patients treated with spesolimab and 56% of those receiving placebo after one week. Infections were reported by 17% and 6% of patients in the spesolimab and placebo groups respectively (at week one). Serious adverse events were reported in 6% of patients treated with spesolimab (at week one).1
In common with other rare diseases, people living with GPP often do not receive a correct diagnosis and their symptoms identified as other forms of psoriasis. Recently, a Global Consensus Delphi Panel of experts concluded a systematic literature review that classified GPP as phenotypically, genetically, immunologically and histopathologically distinct from psoriasis vulgaris / plaque psoriasis. Gaining consensus on definitions, diagnosis and treatment goals is a positive advance to improve patient care.2
For the full press release and link to ‘Notes to Editors’ please click here: https://www.boehringer-ingelheim.com/human-health/skin-diseases/gpp/chmp-recommends-conditional-approval-for-first-gpp-treatment
* A conditional marketing authorization is granted to a medicinal product that fulfils an unmet medical need when the benefit to public health of immediate availability outweighs the risk inherent in the fact that additional data are still required. The marketing authorization holder is expected to provide comprehensive clinical data at a later stage. In this regard, Boehringer Ingelheim will provide additional data on the treatment of subsequent flares.
View source version on businesswire.com: https://www.businesswire.com/news/home/20221014005235/en/
CONTACT:
Boehringer Ingelheim
Corporate Communications
Media + PR
Dr Petra Kienle
Phone: +49 (6132) 77-143877
Fax: +49 (6132) 77 6601
Email: press@boehringer-ingelheim.com
Additional information
www.boehringer-ingelheim.com
About generalized pustular psoriasis (GPP)































































































